Best Chemistry Courses
All topics are curated by you, the community. Free from editorial bias, 100% independent. Upvote your favorite items, propose missing ones and shape the ranking of this topic.
Choosing the right chemistry courses can be difficult, especially if you are not sure what you want to do with your degree. However, with a little research, you can find the perfect course of study to suit your needs.
There are many different types of chemistry, and each one has its own unique application. Whether you want to study the chemical makeup of the universe or the reactions that occur in everyday life, there is a course out there for you.
-
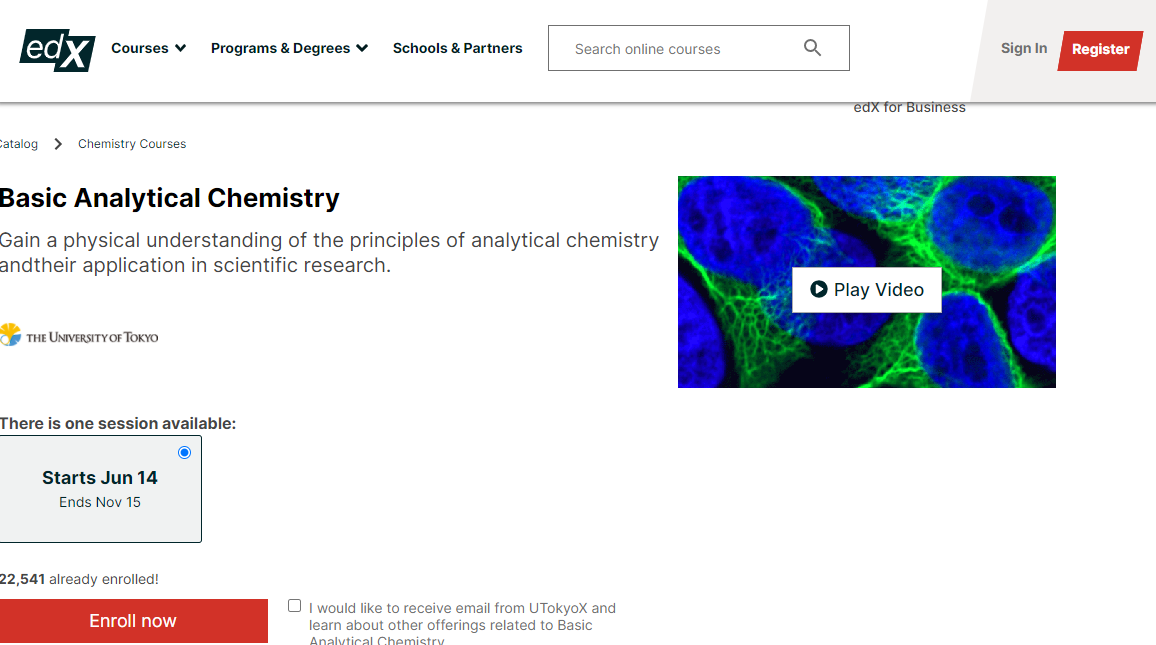
Gain a physical understanding of the principles of analytical chemistry and their application in scientific research.
About this course:
Analytical chemistry takes a prominent position among all fields of experimental sciences, ranging from fundamental studies of Nature to industrial or clinical applications.Analytical chemistry covers the fundamentals of experimental and analytical methods and the role of chemistry around us.
This course introduces the principles of analytical chemistry and provides how these principles are applied in chemistry and related disciplines - especially in life sciences, environmental sciences and geochemistry.
This course, regardless of your background, will teach you fundamental analytical concepts and their practical applications.
By the end of the course, you will deeply understand analytical methodologies in a systematic manner. Finally, this course will help you develop critical, independent reasoning that you can apply to new problems in chemistry and its related fields.
This course is for anyone interested in analytical sciences.
What you'll learn:
- A basic background in the chemical principles, particularly important to analytical chemistry
- The ability to judge the accuracy and precision of experimental data and to show how these judgments can be sharpened by the application of statistical methods
- A wide range of techniques that are useful in modern analytical chemistry
- The skills needed to solve analytical problems in a quantitative manner
- Laboratory skills to obtain high-quality analytical data
Syllabus
Module 1: Basic Tools of Analytical Methods
1. Chemical Measurements and Analytical tools
2. Experimental Error
3. Statistics and Quality Assurance
4. Chemical Equilibrium
5. Sample Preparation
Module 2: Chemical Equilibria for Quantitative Analysis
6. Gravimetric Analysis
7. Effects of Electrolytes
8. Systematic Treatment
9. Monoprotic Acid-Base Equilibria
10. Polyprotic Acid-Base Equilibria
11. Acid-Base Titrations
12. EDTA Titrations
Module 3: Electrochemical Analysis and Spectrophotometry
13. Fundamentals of Electrochemistry
14. Potentiometry
15. Redox Titrations
16. Fundamentals of Spectrophotometry
17. Applications of Spectrophotometry
18. Spectrophotometers
Module 4: Spectrochemical Analysis and Analytical Separations
19. Atomic Spectroscopy
20. Mass Spectrometry
21. Introduction to Analytical Separations
22. Gas Chromatography
23. High-Performance Liquid Chromatography
24. Electrophoresis Analyses
Article from: https://www.edx.org/course/basic-analytical-chemistry
-
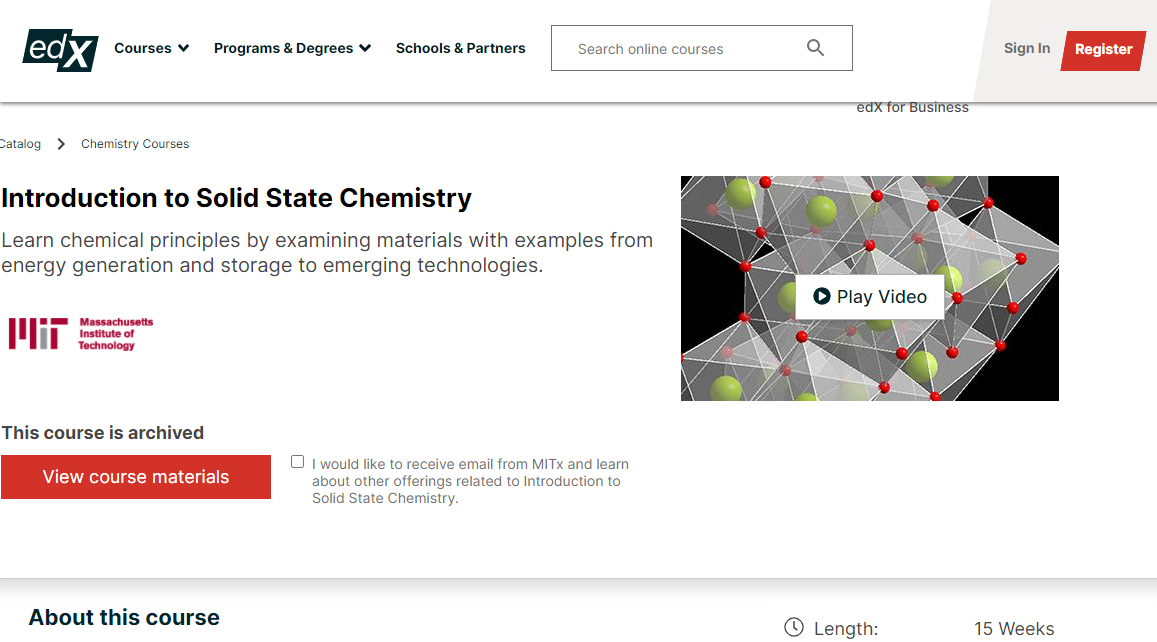
Learn chemical principles by examining materials with examples from energy generation and storage to emerging technologies.
About this course
This first-year University chemistry course explores the basic principles of the chemical bond by studying the properties of solids. Properties such as stiffness, electrical conductivity, thermal expansion, strength, and optical properties are the vehicle by which you can learn a great deal of practical chemistry. AP Chemistry High School students and teachers are among the thousands of learners who have successfully taken the course and enjoyed it.
You will see how experts use their knowledge of trends in the periodic table to predict the properties of materials. 3.091x is an engineering course so there is an emphasis on applications and how materials are used. The on-campus version of the course has been taught for over forty years and is one of the largest classes at MIT.
This course will cover the relationship between electronic structure, chemical bonding, and atomic order, and characterization of atomic arrangements in crystalline and amorphous solids: metals, ceramics, semiconductors, and polymers (including proteins). There will be topical coverage of organic chemistry, solution chemistry, acid-base equilibria, electrochemistry, biochemistry, chemical kinetics, diffusion, and phase diagrams. Examples will be drawn from industrial practice (including the environmental impact of chemical processes), from energy generation and storage (e.g., batteries and fuel cells), and from emerging technologies (e.g., photonic and biomedical devices).
What you'll learn
- You will develop your “chemical intuition”
- A quantitative understanding of chemical principles
- Understanding of crystal structure and its relationship to properties
- Materials properties such as conductivity, optical transmission, stiffness, thermal expansion, and strength
- An understanding of electronic structure, chemical bonding, and atomic order and arrangements
Who can take this course?
Unfortunately, learners residing in one or more of the following countries or regions will not be able to register for this course: Iran, Cuba and the Crimea region of Ukraine. While edX has sought licenses from the U.S. Office of Foreign Assets Control (OFAC) to offer our courses to learners in these countries and regions, the licenses we have received are not broad enough to allow us to offer this course in all locations. edX truly regrets that U.S. sanctions prevent us from offering all of our courses to everyone, no matter where they live.
Article from: https://www.edx.org/course/introduction-to-solid-state-chemistry
-
Offered By University of Kentucky
About this Course
This course is designed to cover subjects in advanced high school chemistry courses, correlating to the standard topics as established by the American Chemical Society. This course is a precursor to the Advanced Chemistry Coursera course. Areas that are covered include atomic structure, periodic trends, compounds, reactions and stoichiometry, bonding, and thermochemistry.
Article from: https://www.coursera.org/learn/chemistry-1#instructors
-
Offered By University of Manchester
About this Course
Chemical reactions underpin the production of pretty much everything in our modern world. But, what is the driving force behind reactions? Why do some reactions occur over geological time scales whilst others are so fast that we need femtosecond-pulsed lasers to study them? Ultimately, what is going on at the atomic level? Discover the answers to such fundamental questions and more on this course in introductory physical chemistry.
The course covers the key concepts of three of the principal topics in first-year undergraduate physical chemistry: thermodynamics, kinetics and quantum mechanics. These three topics cover whether or not reactions occur, how fast they go and what is actually going on at the sub-atomic scale.
Article from: https://www.coursera.org/learn/physical-chemistry
-
A comprehensive course in basic fundamental concepts of Chemistry covering everything for future exams in Chemistry.
What you'll learn:
- Know exactly what is Matter, Element and Compound.
- Learn the scientific methods and type of Mixtures.
- Know the Three State of Maters and Units of Measurements.
- Calculate Scientific Notation and Significant Figures.
- Determine if the results you obtained are Accurate and Precise.
- Understand the Units and Dimensional Analysis.
- Understand the Atomic Theory and Models of the Atom.
- know the Law of Conservation and law of Multiple Proportions.
- Learn Atomic Mass, Mass Number and Isotopes.
- Know how elements are organized in the Periodic Table.
- Learn how to name the Ionic Compounds.
- Know how to write Chemical equations.
- Learn how to make a Chemical Balance.
- Understand The Mole Concept.
- Be able to do Mole calculations successfully.
- Learn determination of Chemical formulas.
- Understand the Stiochiometry.
- To calculate the Limiting Reactant and the Yield of the reaction.
- Study all the Solubility Rules
- Understand the Electrolyte and Non-Electrolyte solutions
- Know all the Types of Chemical Reactions
- Be able to do Calculations on Molar Concentration and Dilution Analysis
- Understand the Gravimetric Analysis and the Titration
- Understand Boyle’s Law, Charles’s Law, Gay-Lussac’s Law, Avogadro’s Law, Combined Gas Law, The Ideal Gas Law, Real Gases, Gas Density and Gas Stoichiometry.
- You will understand Thermochemistry the study of heat change in chemical reactions.
- You will be able to distinguish between Exothermic & Endothermic reactions.
- You will learn Enthalpy and the First Law of Thermodynamics.
- You will be able to calculate Heat Capacity (C) for any element.
- Learn how to calculate standard enthalpy of reaction (ΔH0) for any chemical reaction.
- You will understand the Properties of Waves.
- You will know how Bohr’s Model of the Atom works.
- Understand Schrodinger Wave Equation.
- Be able to find the four quantum numbers in Schrodinger Equation.
- You will be bale to write the Electron configuration for any element.
- Understand the Ground State Electron Configurations and Classification of Elements.
- know the Electron Configurations of Cations and Anions.
- You will learn The Atomic Radius, The Ionization Energy, Effective nuclear charge (Zeff) and The Electron Affinity.
- You will be able to understand Groups Elements Properties.
- You will be able to understand the difference between ionic bond and covalent bond.
- You will understand electronegativity and formal charge.
- You will be able to write Lewis structure.
- You will understand the VSEPR theory.
- You will understand the Hybridization.
Who this course is for:
This course is for High School Students, Chemists, Pharmacy student, biology student, Nursing student and Engineering student,under graduated or graduated.
Everyone who has a big passion with chemistry and want to discover more about it.
Section 1 - Matter and Measurement
- An Introduction To Chemistry
- Methods Of Science
- Categories Of Science
- Steps Of Scientific Method
- Theory And Law
- The Scientific Method
- Law of Conservation of Mass
- Matter, Element And Compound
- Types Of Mixtures
- The Three States Of matter
- Properties And Changes Of matter
- Units Of Measurements
- Scientific Notations
- Significant Figures
- Accuracy And Precession
- Units and Dimensional Analysis
Section 2 - Atoms, Molecules, and Ions
- Dalton’s Atomic Theory
- Atomic Symbols and Models
- Law of Conservation of Mass
- Law of multiple proportions
- The Structure of The Atom
- Discovery of the Electron
- The nuclear model of the atom
- Atomic Mass, Mass Number and Isotopes
- Mass number and Atomic Masse
- Periodic Table of the Elements
- Metals, Nonmetals, and Metalloids
- Molecular Substances
- Ionic Substances
- Organic Compounds
- Ionic Compounds
- Rules for predicting Charges
- Naming of Ionic Compounds
- Formula of Ionic Compounds
- Naming of Binary Molecular Compounds
- Acids and Corresponding Anions
- Naming of Hydrates
- Writing Chemical Equations
- Balancing Chemical Equations
Section 3 - Calculations With Chemical Formulas And Equations
- Introduction to Calculations in Chemistry
- Mass and Moles of Substance
- Molecular Mass and Formula Mass
- The Mole Concept
- Mole Calculations
- Determining Chemical Formulas
- Elemental Analysis, Percentage of C, H and O
- Determining Formulas
- Molecular Formula from Empirical Formula
- Stoichiometry: Quantitative Relations in Chemical Reactions
- Limiting Reactant
- Theoretical Yield
Section 4 – Chemical Reactions
- Introduction to Chemical Reactions
- Ionic Theory of Solutions and Solubility Rules
- Electrolytes and Non-electrolytes
- Solubility Rules
- Molecular and Ionic Equations
- Acid Base, Neutralization and Oxidation-Reduction Reactions
- Oxidation number
- Types of Oxidation-Reduction Reactions
- Working with solutions
- Quantitative Analysis
Section 5 – Gaseous State
- Introduction to the Gaseous State
- Gas Pressure and Its Measurement
- Boyle’s Law: Relating Volume and Pressure
- Charles’s Law: Relating Volume and Temperature
- Gay-Lussac’s Law : Relating Pressure and Temperature
- Combined Gas Law : Relating Pressure, Temperature and Volume
- Avogadro’s Law: Relating Volume and Amount
- The Ideal Gas Law
- Standard Temperature and Pressure (STP)
- Gas Density; Molecular-Mass Determination
- Gas Stoichiometry
- Gas Mixtures; Law of Partial Pressures
- Real Gases
Section 6 – Thermochemistry
- Introduction to Thermochemistry
- Thermodynamics
- Thermodynamic Equations
- Heat Capacity (C)
- Standard Enthalpy of Formation ΔH formation
Section 7 – Quantum Theory and the Electronic Structure of Atoms
- Properties of Waves
- Bohr’s Model of the Atom
- Schrodinger Wave Equation
- Aufbau principle and Hund's Rule
- Electron configuration
Section 8 – Periodic Relationships
- Ground State Electron Configurations and Classification of Elements
- Electron Configurations of Cations and Anions
- Effective nuclear charge (Zeff)
- The Atomic Radius
- The Ionization Energy
- The Electron Affinity
- Groups Elements Properties
Section 9 – Ionic and Covalent Bonding
- The Ionic and Covalent Bonding
- Electronegativity and Polar Covalent Bond
- Writing Lewis Structures
- Formal Charge
Section 10 – Molecular Geometry and Chemical Bonding Theory
- Valence shell electron pair repulsion (VSEPR) model
- Dipole Moments and Polar Molecules
- Valence Bond Theory
- Hybridization
In addition to that, there are a lot of QUIZZES added to this course in order to enhance students understanding to the contents of this course and get the desired value !!!
For example in One Quiz you will practice on solving the following questions:
- Can an element be broken down into a simpler substance?
- What is a compound?
- What is a mixture?
- How many classifications of mixtures are there?
- Mixtures can be separated. Which of the following represents a way a mixture cannot be separated?
- Which of the following is a compound?
- Which of the following is an element?
- Are there more compounds or more elements?
- Which of the following is not a mixture?
- Can compounds be separated?
This Course contains the following Quizzes:
Elements, Compounds and Mixtures
Properties and Changes of Matter
Scientific Notation
Significant Figures
Periodic Table of The Elements
Naming an Ionic Compound from Its Formula
Balancing Chemical Equations
Mole Calculation for atoms, molecules, units and ions
Calculate the percentage composition
Limiting Reactant
Classification of Solutes in Aqueous Solution
Solubility Rules for Ionic Compounds
Types of Oxidation-Reduction Reactions
Oxidation Numbers
Boyle’s Law
Charles's law
Gay-Lussac's
Combined Gas Law
The Ideal Gas Law
Gas Stoichiometry
Exothermic and Endothermic
Enthalpy
Hess law
Properties of waves
Bohr’s Model
Electron Configurations
Electron Configurations of Cations and Anions
The Atomic Radius
Ionization energy
Covalent bonds, Ionic bonds and Polar covalent bonds
Formal Charge (FC)
Article from: https://www.udemy.com/course/general-chemistry-101-chapter-1-matter-measurment/
-
A condensed course for high school, college, MCAT, DAT or AP chemistry.
What you'll learn:
Students will have expert level understanding of general chemistry by the end of this course.
Requirements
- A basic understanding of high school math is required.
Description
This is a condensed general chemistry course. It takes all of the major topics in a general chemistry course and condenses them into understandable bite-sized animated video chunks. It is great for high school or college students and anyone studying for a major exam like the MCAT, DAT or AP chemistry exam. The course is condensed into 11 understandable lectures, with over 40 lectures.
Who this course is for:
Anyone in college or high school chemistry or who has an interest in chemistry.
Article from: https://www.udemy.com/course/general-chemistry-condensed-course-college-or-high-school/
-
An introductory course for anybody who wants to study chemistry - either towards future pathways or just for fun!
What you'll learn:
By the end of this course you will have received a thorough preparation in the principles of Chemistry. If you follow the course carefully the you should be looking
Requirements
- There are no essential course requirements, just a desire to learn more about Chemistry and a willingness to make the necessary effort!
Description:
This course is a comprehensive introduction to Chemistry, covering everything you will need to know as you prepare for possible future exams. It doesn't matter how much, or how little, prior knowledge of Chemistry you've got as this course will take you through all the necessary stages.
The content is based on the International GCSE specification as followed by thousands of students around the world, and will prepare you thoroughly for progression to advanced programmes.
In Section 1, The Principles of Chemistry, there are five video lessons, each one lasting about 20 minutes. The first lesson introduces the states of matter and atomic structure. In the next lesson we progress to relative formula mass and chemical equations - don't be put off by these as everything is very carefully explained! Lesson 3 covers chemical formulae, and we progress in lesson four to look at reacting masses, ionic bonding and covalent bonding. Lesson five wraps up this part of the course with metallic crystals and electrolysis.
Who this course is for:
- This course is intended for :
- students who wish to follow a career in the sciences or medicine and who require really clear chemistry instruction
- students who may already be following a course but who need additional support in order to successfully progress
- anybody with a genuine interest in learning about the first principles of Chemistry!
Article from: https://www.udemy.com/course/chemistry-101-part-1-principles-of-chemistry/
-
Develop your knowledge of chemistry to support your pupils' learning as part of the primary science curriculum.
Improve the way you teach primary science by building your chemistry knowledge
To teach primary science effectively teachers must have strong subject knowledge, but many primary teachers are not science experts.
On this course, you’ll explore the key knowledge required to teach the chemistry elements of the primary science curriculum.
You’ll learn about the common misconceptions and misunderstandings children may have and learn how you can help your students identify and overcome them.
You’ll discover practical activities to do with your primary school students that will help them to learn scientific concepts in a fun and engaging way.
What topics will you cover?
- Why good subject knowledge is essential when teaching primary science.
- Developing subject knowledge for the chemistry in the primary science.
- Understanding children’s misconceptions and identifying teaching strategies to identify and overcome them.
- Curriculum topics of: understanding materials and their properties; states of matter; changes of materials.
Who is this accredited by?
The CPD Certification Service: The CPD Certification Service was established in 1996 and is the leading independent CPD accreditation institution operating across industry sectors to complement the CPD policies of professional and academic bodies.
Learning on this course
On every step of the course you can meet other learners, share your ideas and join in with active discussions in the comments.
What will you achieve?
By the end of the course, you‘ll be able to...
- Identify the importance of good subject knowledge when teaching primary science.
- Develop personal subject knowledge of chemistry.
- Apply subject knowledge to class teaching of pupils aged 5-11 years old.
- Explore practical activities to teach scientific concepts in the primary curriculum.
- Identify common misconceptions children may have in primary science concepts.
- Develop teaching strategies to identify and overcome children’s misconceptions.
Who is the course for?
This course is designed for primary teachers and trainee teachers in primary education (pupils aged 5-11 years) who want to develop their chemistry subject knowledge.
The course refers to the English National Curriculum, but the subject matter is equally transferable to other curricula, and you’ll be encouraged to explore connections with your own national curriculum.
What's included?
This is a premium course. These courses are designed for professionals from specific industries looking to learn with a smaller group of like-minded individuals.
- Unlimited access to this course
- Includes any articles, videos, peer reviews and quizzes
- Certificate of Achievement to prove your success when you're eligible
- Download and print your Certificate of Achievement anytime
https://www.futurelearn.com/courses/teaching-primary-science-chemistry